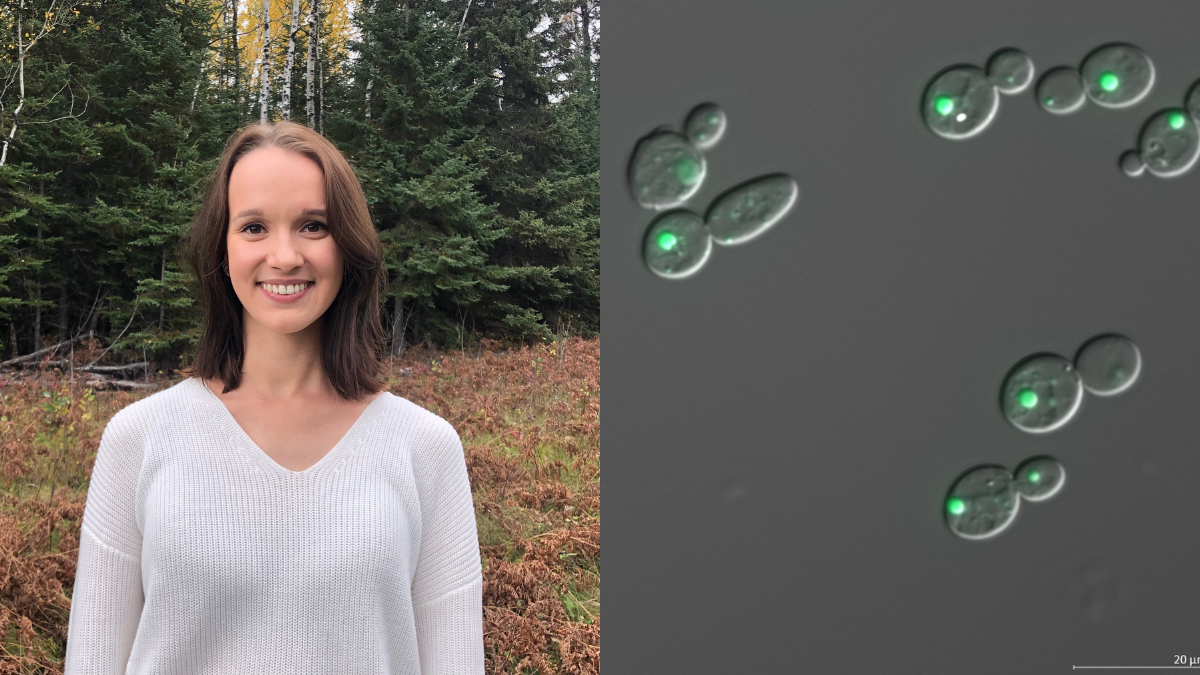
Emily Puumala (left) is a PhD student whose research focuses on identifying and characterizing new antifungal compounds to combat drug resistance in the fungal pathogen Candida auris (right).
November 21, 2022
By Betty Zou
As the global health and research communities mark World Antimicrobial Awareness Week, members of the Emerging and Pandemic Infections Consortium (EPIC) are making strides in the fight against antimicrobial resistance.
While much of the conversation about antimicrobial resistance centres around antibiotics and bacteria, drug-resistant fungal pathogens are becoming an increasing threat. In October, the World Health Organization released its first ever list of fungal priority pathogens, which included four “critical priority” fungi.
One of the four is the highly drug-resistant Candida auris, an emerging fungal pathogen first discovered in humans in 2009. Nine in ten isolates of Candida auris are resistant to at least one class of antifungal drugs, making infections with this pathogen especially difficult to treat.
“Four per cent of Candida auris isolated from patients are resistant to all classes of antifungal drugs,” said Nicole Robbins, a senior research associate in Leah Cowen’s lab at the University of Toronto. “For those patients, there are no treatment options left.”
“We were interested in identifying therapeutic strategies that were more effective than the existing antifungals we had in the clinic,” said Emily Puumala, a PhD student in Cowen’s group and the first author of a new paper that describes a novel way in which fungal pathogens like Candida auris can develop drug resistance.
The researchers started by searching a library of 400 drug-like molecules to find ones that were effective against Candida auris, including one that also blocked the growth of Candida albicans, Cryptococcus neoformans and Aspergillus fumigatus, the other three critical priority fungal pathogens on the WHO list.
Honing in on the most potent molecule in the collection, Puumala and her colleagues showed that it works by disrupting the balance of molecules called lipids which, in turn, affects the integrity of the membrane that surrounds the cell.
By trying to dissect how the compound works using the model yeast Saccharomyces cerevisiae, the researchers also discovered a new way through which fungal pathogens become resistant to antifungal drugs. In response to the lipid imbalance caused by the antifungal molecule, S. cerevisiae relies on a molecular pathway involving the proteins Hal9 and Hsp12 to dampen the stress caused to the cell membrane.
“These findings are exciting in that they highlight the power of interdisciplinary approaches to uncover new facets of fungal biology and vulnerabilities in fungal pathogens that can be exploited for the development of much-needed antifungal drugs,” said Cowen, a professor of molecular genetics in the Temerty Faculty of Medicine and U of T’s vice-president, research and innovation, and strategic initiatives.
Cowen’s team is now turning their attention to some of the other molecules in the library that also showed antifungal activity. By learning more about how different compounds attack fungal pathogens and how pathogens respond, they hope to uncover strategies to prevent and lessen the impact of antimicrobial resistance.
Alongside these advances in our understanding of how resistance develops, EPIC members are addressing one of the biggest challenges of antimicrobial stewardship – knowing what pathogen you’re up against and what drugs will work against it.
“Early identification of resistant organisms is critical to both treatment and mitigating spread,” said Samira Mubareka, a clinician scientist at Sunnybrook Research Institute and a medical microbiologist at Sunnybrook Health Sciences Centre who sees drug-resistant infections daily.
“Rapid diagnostics are an important tool in the fight against antimicrobial resistance. Current approaches can take up to several days depending on the situation, potentially delaying diagnosis and management.”
Without knowing which microbe is causing the infection and which drugs would be most effective, doctors can end up prescribing the wrong type or dose of antibiotics, contributing to unnecessary antibiotic exposures that hasten the development of drug resistance.
To address this gap, Mubareka, who is also an associate professor in the department of laboratory medicine and pathobiology at U of T, teamed up with Warren Chan, professor and director of the Institute of Biomedical Engineering, to validate a new tool for diagnosing infections.
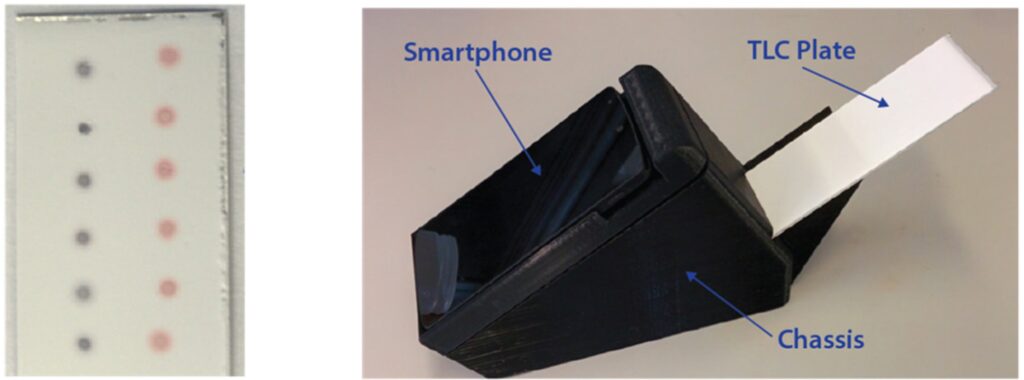
The tool is the culmination of 20-years of work by Chan and his group to develop nanotechnologies that can improve infectious disease diagnostics. It works by pairing gold nanoparticles with short snippets of DNA that recognize unique pathogen-specific genes or genes that confer antibiotic resistance. If the matching DNA is present, the gold nanoparticle-DNA binds to it and causes the solution to change from purple to red.
Through collaborations with Mubareka and fellow EPIC member Karen Maxwell, an associate professor in the department of biochemistry at U of T, Chan and his team have shown that this simple test can discern whether a patient is infected with influenza virus or SARS-CoV-2 and identify which bacteria is causing a patient’s urinary tract infection. Using clinical samples and patient swabs, the researchers also demonstrated that the test can diagnose antibiotic resistance with 90% sensitivity and 95% specificity, which is as good as or better than standard practices used today.
Importantly, the nanoparticle-based test produces results in three hours without the need for highly specialized equipment or skilled technicians. In contrast, current approaches often involve PCR tests — which require expensive equipment and reagents and specially trained staff — and growing the pathogen in a lab to test its sensitivity to different drugs, which can take 20 to 72 hours.
“It took us 20 years to get the chemistry right and to build the system,” said Chan. “Now we’re trying to put it together into a simple package that’s easy to use by anyone, anywhere. How do we make it practical and accessible for the broader community?”
Part of their solution involves developing a smartphone platform that can run the gold nanoparticle-based tests and analyze and transmit the results to a central location for public health tracking. The researchers also did a cost analysis and determined that, using 3D printing and low-cost components, the device could be manufactured for roughly $32 and each test could cost as low as $0.38.
The affordability and ease-of-use of these rapid diagnostic tests hold tremendous potential for improving antimicrobial-prescribing practices around the world, especially in low-resource settings where access to lab-based testing is difficult and can take a long time. To bring their innovations to market, Chan and his former PhD student Kyryl Zagorovsky founded a start-up company and are working to scale up manufacturing of the gold nanoparticle test components and devices.
“As an engineer, we try to build solutions that are useful to society,” said Chan. “Ultimately, what we want is for people to use what we’ve developed.”